what is reverse osmosis :
![]()
|
What Is. . . Reverse Osmosis Anyone who has been through a high school science class will likely be familiar with the term osmosis. The process was first described by a French Scientist in 1748, who noted that water spontaneously diffused through a pig bladder membrane into alcohol. Over 200 years later, a modification of this process known as reverse osmosis allows people throughout the world to affordably convert undesirable water into water that is virtually free of health or aesthetic contaminants. Reverse osmosis systems can be found providing treated water from the kitchen counter in a private residence to installations used in manned spacecraft.
Reverse Osmosis is a technology that is found virtually anywhere pure water is needed; common uses include:
How Reverse Osmosis Works
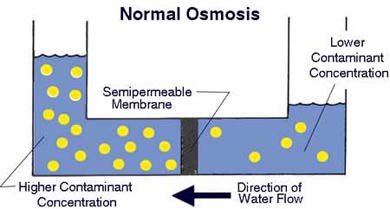
A semipermeable membrane, like the membrane of a cell wall or a bladder, is selective about what it allows to pass through, and what it prevents from passing. These membranes in general pass water very easily because of its small molecular size; but also prevent many other contaminants from passing by trapping them. Water will typically be present on both sides of the membrane, with each side having a different concentration of dissolved minerals. Since the water i the less concentrated solution seeks to dilute the more concentrated solution, water will pass through the membrane from the lower concentration side to the greater concentration side. Eventually, osmotic pressure (seen in the diagram below as the pressure created by the difference in water levels) will counter the diffusion process exactly, and an equilibrium will form.
The process of reverse osmosis forces water with a greater concentration of contaminants (the source water) into a tank containing water with an extremely low concentration of contaminants (the processed water). High water pressure on the source side is used to "reverse" the natural osmotic process, with the semi-permeable membrane still permitting the passage of water while rejecting most of the other contaminants. The specific process through which this occurs is called ion exclusion, in which a concentration of ions at the membrane surface from a barrier that allows other water molecules to pass through while excluding other substances.
Semipermeable membranes have come a long way from the natural pig bladders used in the earlier osmosis experiments. Before the 1960's, these membranes were too inefficient, expensive, and unreliable for practical applications outside the laboratory. Modern advances in synthetic materials have generally solved these problems, allowing membranes to become highly efficient at rejecting contaminants, and making them tough enough to withstand the greater pressures necessary for efficient operation.
Even with these advances, the "reject" water on the source side of a Reverse Osmosis (RO) system must be periodically flushed in order to keep it from becoming so concentrated that it forms a scale on the membrane itself. RO systems also typically require a carbon prefilter for the reduction of chlorine, which can damage an RO membrane; and a sediment prefilter is always required to ensure that fine suspended materials in the source water do not permanently clog the membrane. Hardness reduction, either through the use of water softening for residential units or chemical softening for industrial use, may also be desirable in hard water areas.
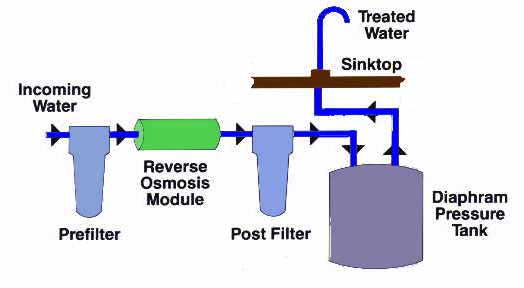
Low Pressure (Residential) Systems Low pressure RO systems generally refer to those systems with a water feed pressure of less than 100 psig. These are the typical countertop or undersink residential systems that rely primarily on the natural water pressure to make the reverse osmosis process function; a typical system is shown schematically below.
Typical Point of Use Reverse Osmosis System Countertop units typically have an unpressurized storage tank; Undersink units typically have a pressurized accumulator storage tank where the water pressure tends to increase as the tank fills. This pressurized system provides sufficient pressure to move the water from the undersink storage tank to the faucet. Unfortunately, this also creates a back pressure against the membrane, which can decrease its efficiency. Some units overcome this by using unpressurized tanks with a pump to get the treated water where it is needed.
Low pressure units typically provide between 24 and 35 gallons per day of water (Pure-Pro System offer 50-80 gallons per day), Water purity can be as high as 95 percent of rejection. These systems can be highly affordable, undersink units starting at about US $500. These units produce water for a cost as low as five cents per gallon once maintenance and water costs are factored in. Maintenance usually requires replacing any pre- or postfilters (typically one to four times per year); and the reverse osmosis cartridge once every two to three years, depending on usage. Look for PPW Home Reverse Osmosis System to find products that have been successfully tested to industry performance standards; and to Certified Water Specialists (CWS I-V), Certified Sales Representatives (CSR), and Certified Installers (CI) for advice on your water needs, and equipment installation.
High Pressure (Commercial/Industrial) Systems High pressure systems typically operate at pressures between 100 and 1000 psig, depending on the membranes chosen and the water being treated. These systems are usually used in industrial or commercial applications where large volumes of treated water are required at a high level of purity. Most commercial and industrial systems use multiple membranes arranged in parallel to provide the required quantity of water. The processed water from the first stage of treatment can then be passed through additional membrane modules to achieve greater levels of treatment for the finished water. The reject water can also be directed into successive membrane modules for greater efficiency (see diagram below), though flushing will still be required when concentrations reach a level where fouling is likely to occur.
High pressure industrial units typically provide from 10 gallons to thousands of gallons per day of water with an efficiency of 1-9 gallons of reject water per gallon of treated water. Water purity can be as high as 95 percent. These systems tend to be larger and more complicated than low pressure systems, and this is reflected in their costs, which range from US $1000 through tens of thousands of dollars for a large, multi-module unit capable of providing desalinated drinking water for a resort facility or water bottling plant.
What Reverse Osmosis Treats Reverse osmosis can treat for a wide variety of health and aesthetic contaminants. Effectively designed, RO equipment can treat for a wide variety of aesthetic contaminants that cause unpleasant taste, color, and odor problems like a salty or soda taste caused by chlorides or sulfates. RO can also be effective for treating health contaminants like arsenic, asbestos, atrazine (herbicides/pesticides). fluoride, lead, mercury, nitrate, and radium. When using appropriate carbon prefiltering (commonly included with most RO systems), additional treatment can also be provided for such "volatile" contaminants as benzene, trichloroethylene, trihalomethanes, and radon. Some RO equipment is also capable of treating for biological contaminants like Cryptosporidium. The Water Quality Association (WQA) cautions, however, that while RO membranes typically remove virtually all known microorganisms and most other health contaminants, design consderations may prevent a unit from offering foolproof protection when incorporated into a consumer drinking water system. When looking for a product to treat for a given health contaminant, care should be used to find products that have been tested successfully for such purposes at a quality testing laboratory.
Conclusion Reverse osmosis is a relatively new, but very effective, application of an established scientific process. Whether it is used to meet the needs of a typical family of four, or the needs of an industrial operation requiring thousands of gallons per day, it can be a cost effective to provide the required quantity of highly treated water. With continual advances in system and membrane design that boost efficiency and reliability, RO can be expected to play a major role in water treatment for years to come.
|
![]()